c2h2 lewis dot structure|How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) : Cebu How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) It is helpful if you: Try to draw the C 2 H 2 Lewis structure before watching the video. Watch the video and . If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
c2h2 lewis dot structure,2.1K. 284K views 10 years ago. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene). For the C2H2 structure use the periodic table to find the total. In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like. Learn how to draw the Lewis structure of acetylene (C2H2), a linear and non-polar molecule with a triple bond between carbon atoms and a single bond .
How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) It is helpful if you: Try to draw the C 2 H 2 Lewis structure before watching the video. Watch the video and . C2H2 Lewis Dot Structure + Geometry. chem101csub. 3.82K subscribers. Subscribed. 35. 16K views 10 years ago. If you are asking "help me with Lewis structures" then .
This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms and two hydrogen atoms. In this . Learn how to draw the lewis dot structure of C2H2 (or acetylene or ethyne) with 6 simple steps and images. Find out the valence electrons, center atom, .With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Remember that Hydrogen (H) atoms .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a .
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . Chemists normally . C2H2 Lewis Structure. Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule. The electrons that participate in forming bonds are called bonding pairs of electrons while the ones that do not take part in any bond formation are called lone pairs or non-bonding .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .
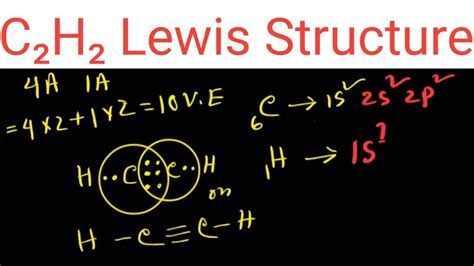
The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., linear. The bonded atoms in C 2 H 2 form a mutual bond angle of 180°. The central C-atoms have sp hybridization in C 2 H 2.
Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure.c2h2 lewis dot structure Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs. Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs.Bonding in Ethane. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry.The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, .The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves representing each atom using its chemical symbol and drawing dots around it to represent its valence electrons.c2h2 lewis dot structure How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne) 5 Steps to Draw the Lewis Structure of C2H2 Step #1: Calculate the total number of valence electrons. Here, the given molecule is CH2. In order to draw the lewis structure of CH2, first of all you have to find the total number of valence electrons present in the CH2 molecule. (Valence electrons are the number of electrons present in the .

Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon .
The Lewis structure of C 2 H 2 is a highly unreactive and stable compound with one single bond and one triple bond in it, as well as fulfilling all the rules of octave rules, making it more stable. The Lewis structure also known as the dot structure of an acetylene or ethyne molecule is mainly given to represent the arrangement of valence .We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of .
Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Suggest Corrections. 35. Q. Draw the electron dot structure of ethyne and also draw its. structural formula. Q. Question 30. Drawthe electron dot structure of ethyne and also draw its structural formula. Q. Draw electron dot structure of ethyne. Q.

PROBLEM 4.2.4 4.2. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Correct Correct Wrong. CCl 4 (A refrigerant) C 2 H 6 (Ethane) CHClCH (Bad layout, hydrogen and chlorine are central atoms) Note: Electronegativity values: C = 2.55; Cl = 3.16; H = 2.20. Step 2: Add up the valence electrons for each atom in the molecule. For example, H 2 O 2 H: 2 x 1 electron = 2 electrons. 1 O: 1 x 6 electrons = 6 electrons. Steps for Writing Lewis Structures. Example 3.4.1 3.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a .We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with .
c2h2 lewis dot structure|How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH0 · Lewis Structure of C2H2 (With 6 Simple Steps to Draw!)
PH1 · Lewis Structure for C2H2 (Ethyne)
PH2 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH3 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH4 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond
PH5 · C2H2 Lewis Structure, Molecular Geometry
PH6 · C2H2 Lewis Structure Tutorial
PH7 · C2H2 Lewis Structure
PH8 · C2H2 Lewis Dot Structure + Geometry
PH9 · C2H2 (Ethyne) Lewis structure
PH10 · C2H2 (Acetylene